Immune evasion and provocation by Mycobacterium tuberculosis - Nature.com
Abstract
Mycobacterium tuberculosis, the causative agent of tuberculosis, has infected humans for millennia. M. tuberculosis is well adapted to establish infection, persist in the face of the host immune response and be transmitted to uninfected individuals. Its ability to complete this infection cycle depends on it both evading and taking advantage of host immune responses. The outcome of M. tuberculosis infection is often a state of equilibrium characterized by immunological control and bacterial persistence. Recent data have highlighted the diverse cell populations that respond to M. tuberculosis infection and the dynamic changes in the cellular and intracellular niches of M. tuberculosis during the course of infection. M. tuberculosis possesses an arsenal of protein and lipid effectors that influence macrophage functions and inflammatory responses; however, our understanding of the role that specific bacterial virulence factors play in the context of diverse cellular reservoirs and distinct infection stages is limited. In this Review, we discuss immune evasion and provocation by M. tuberculosis during its infection cycle and describe how a more detailed molecular understanding is crucial to enable the development of novel host-directed therapies, disease biomarkers and effective vaccines.
Introduction
Mycobacterium tuberculosis is a respiratory pathogen that is estimated to infect one-quarter of the world's population, and it has killed more people over the course of human history than any other microorganism. M. tuberculosis is thought to have originated from environmental mycobacteria that entered human populations in the Horn of Africa more than 70,000 years ago, and the global lineages of M. tuberculosis today mirror the subsequent routes of human migration1. Having evolved with humans for millennia, M. tuberculosis is exquisitely well adapted to navigate the human immune system. The life cycle of M. tuberculosis (Fig. 1) depends on its ability to interact with the immune system in seemingly distinct ways: it evades the innate immune response, persists in the face of an adaptive immune response without causing symptomatic disease, and elicits a robust inflammatory response to cause extensive tissue pathology for it to be transmitted. Understanding how M. tuberculosis orchestrates its life cycle is crucial for the design of preventive and therapeutic vaccines, as well as novel therapies and disease biomarkers.
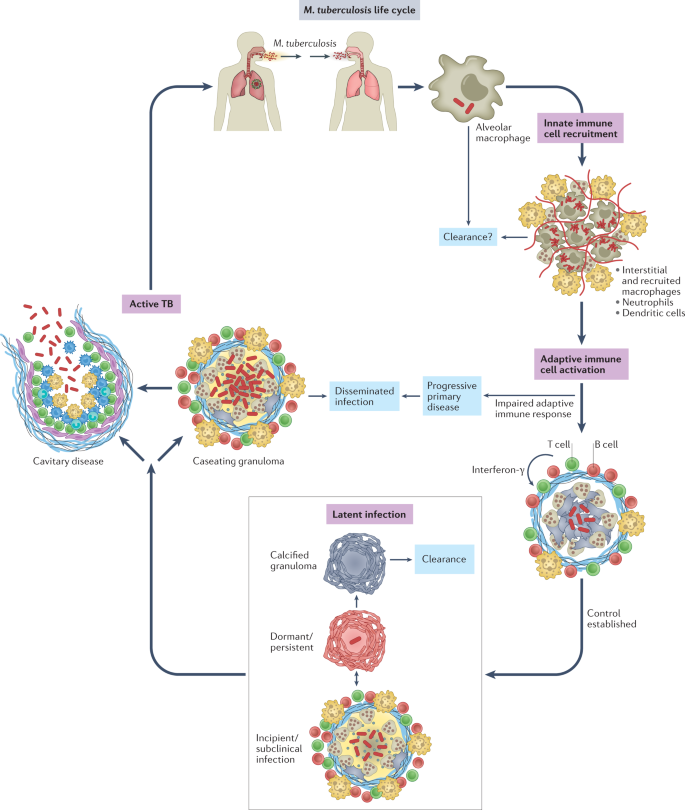
Mycobacterium tuberculosis is transmitted by aerosol from an individual with active pulmonary infection. The first cells infected are alveolar macrophages. After infected alveolar macrophages migrate into the lung interstitium, the bacilli infect a variety of monocyte-derived and tissue-resident macrophages, dendritic cells and neutrophils. Whether the innate immune response clears infection in some individuals is not clear. Dendritic cells travel to the draining lymph node, where antigen-specific T cells are primed. T cells return to the site of infection and are crucial to establish control and prevent dissemination. With an effective adaptive immune response, most infected individuals develop latent infection, a spectrum of outcomes ranging from sterilized infection to subclinical disease. For reasons that are not well understood, ~5–10% of infected individuals will develop active tuberculosis (TB), most often cavitary disease in the lungs. Most transmission occurs from individuals with cavitary pulmonary disease.
A central feature of tuberculosis (TB) pathogenesis is the ability of the causative organism, M. tuberculosis, to survive in diverse intracellular environments within a variety of myeloid cell populations. The outcome of M. tuberculosis infection is shaped by host genetics, co-morbidities, environmental factors and microbial virulence determinants in ways we are just beginning to understand. To establish infection, M. tuberculosis resists and disarms macrophages and neutrophils in the lung, undermining lysosomal trafficking pathways to survive intracellularly. M. tuberculosis infects tissue-resident alveolar macrophages (AMs), which arise during embryogenesis, as well as a variety of phenotypically distinct macrophage populations of haematological origin2,3,4,5,6,7. Infected dendritic cells (DCs) travel to the draining lymph node and prime T cells, which then return to the infected lung. This takes several weeks, but once an effective adaptive immune response develops, T cells, B cells and activated macrophages form the characteristic granuloma, and bacterial control is established. Most often, bacterial replication is contained, and the inflammatory response subsides, leading to latent TB. Individuals with latent TB have an adaptive immune response to M. tuberculosis but no symptoms, culturable bacilli or disease manifestations. Latent TB likely encompasses a spectrum of outcomes that include bacterial elimination and subclinical disease8, although currently there is no way to distinguish individuals who sterilized infection from those that harbour viable bacilli. Approximately 5–10% of infected individuals will go on to develop active TB, due to either progressive primary infection or 'reactivation', which can occur long after initial infection9. In reactivation, TB develops in the setting of a previously 'successful' (although not sterilizing) adaptive immune response. A wide variety of disease manifestations are possible, from cavitary lung disease to focal infection involving nearly any organ system, to widely disseminated infection. Cavitary lung disease is most common, and, importantly, individuals with cavitary lesions are the most infectious. Thus, although disseminated infection can be devastating for the infected individual, it is not a successful outcome for the bacteria either, as it is much less likely to lead to transmission. In this Review, we discuss how M. tuberculosis evades immune-mediated clearance while capitalizing on the host inflammatory response at different phases of its life cycle. We focus on recent studies, highlight gaps in knowledge and consider how our current understanding will inform new therapies, vaccines and diagnostics.
How M. tuberculosis establishes infection
The cellular niche of M. tuberculosis
Understanding the earliest events of infection is crucial for the development of a preventive vaccine. The infectious dose of M. tuberculosis is remarkably low, estimated to be approximately three bacilli, highlighting how effective M. tuberculosis is at evading the innate immune response10. Even before uptake by phagocytic cells, M. tuberculosis encounters alveolar lining fluid (Fig. 2), a complex mixture of lipids and proteins secreted by alveolar epithelial cells, which includes surfactant proteins and hydrolases that interact with mycobacterial surface glycolipids. Alveolar lining fluid enhances pathogen uptake and killing by phagocytes and has a variable impact on interactions with alveolar epithelial cells11,12. Deficiencies in pulmonary surfactant due to inflammageing or smoking promote intracellular M. tuberculosis replication and increase the risk of developing TB12,13. In addition, antibody opsonization may promote innate immune control against M. tuberculosis14. Interestingly, recent data suggest that M. tuberculosis-specific IgM antibodies elicited by vaccination might be protective15. This indicates that it might be possible to develop vaccines that generate humoral responses that disrupt the earliest steps of infection, for example, by neutralizing secreted or cell envelope-associated virulence factors or by functionally altering subsequent macrophage interactions, as discussed further herein.
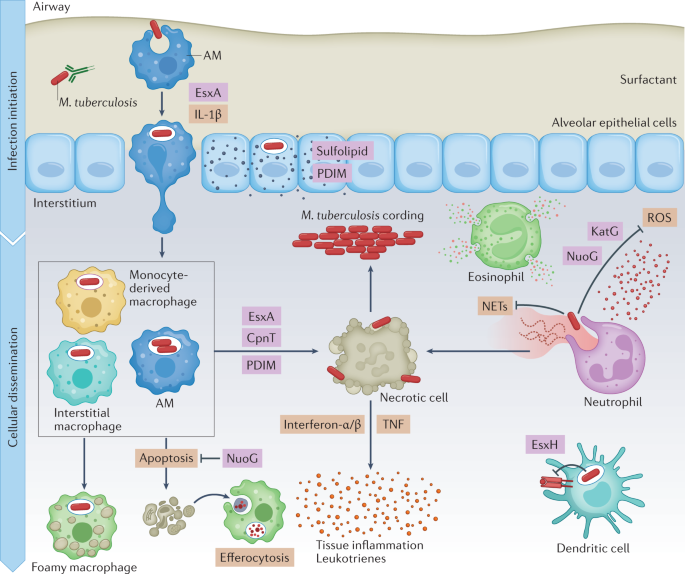
In the airways, Mycobacterium tuberculosis first encounters alveolar macrophages (AMs), which present a permissive niche for infection establishment. In addition, M. tuberculosis infects pulmonary epithelial cells and sheds virulence lipids such as phthiocerol dimycoserosate (PDIM) and sulfolipids into epithelial host cell membranes. Infected AMs migrate into the lung interstitium, in a manner that depends on the ESX-1 secretion system of M. tuberculosis and production of host IL-1β. When M. tuberculosis enters the lung interstitium, it infects additional macrophage populations. Neutrophils respond to M. tuberculosis infection by inducing reactive oxygen species (ROS) and neutrophil extracellular traps (NETs), which do little to control bacterial replication and exacerbate inflammation instead. Some macrophages appear to be better at controlling infection than others, using antimicrobial mechanisms such as phagolysosomal fusion, autophagy and oxidative stress to kill M. tuberculosis, and exhibit a pro-inflammatory metabolic shift. M. tuberculosis detoxifies reactive oxygen with the catalase–peroxidase KatG, and also inhibits ROS production in macrophages and neutrophils using NuoG. When infected macrophages undergo an apoptotic mode of cell death, they can be cleared by efferocytosis, which limits pathogen spread. M. tuberculosis uses virulence factors such as EsxA, CpnT and PDIM to induce necrosis and promote M. tuberculosis dissemination, extracellular replication and immunopathology. M. tuberculosis also induces a foamy macrophage phenotype by enhancing accumulation of host lipids, which support bacterial nutrition and persistence. Host cytokines, such as type I interferons and TNF, and leukotrienes contribute to tissue inflammation, which in turn recruits more cells. Further, M. tuberculosis EsxH suppresses antigen presentation by dendritic cells to delay the onset of adaptive immunity.
Much of our knowledge of the earliest events of infection come from the mouse model. In that model, epithelial cells are infected in the first 48 h after infection; however, M. tuberculosis does not appear to replicate or persist in these cells7. The mycobacterial lipid phthiocerol dimycocerosate (PDIM) can spread into epithelial membranes and modulate immune responses16. Microfold cells (M cells) may also be a portal of entry, allowing M. tuberculosis to gain access to underlying lymphoid tissue in the upper airways17,18. In mice, tissue-resident AMs are the predominant cell type infected during the first 2 weeks7. AMs are located in the air spaces, where they are continually exposed to environmental particulates. In the absence of a microbial challenge, AMs are poised to suppress inflammatory responses to foreign material to prevent lung injury19,20. M. tuberculosis infection of AMs initiates a nuclear factor erythroid 2-related factor 2 (NRF2)-driven antioxidant transcriptional response that correlates with impaired control of M. tuberculosis growth21,22. Ageing may also make AMs more permissive to M. tuberculosis replication23. After 2 weeks, infected AMs migrate out of the alveolar space into the lung interstitium in a process dependent on host IL-1β signalling and an M. tuberculosis type VII secretion system, ESX-1 (ESAT-6 secretion system 1; ESAT-6 is also known as EsxA), which is discussed later. After entering the lung interstitium, M. tuberculosis infects additional phagocytic cell types7. On the basis of its transcriptional responses, M. tuberculosis within AMs appears to be able to access host iron and fatty acids, experiences minimal oxidative and nitrosative stress, and has a high replicative capacity2,4. Moreover, selective depletion of AMs decreases lung M. tuberculosis burden in mice2,24, supporting the idea that AMs are a particularly permissive niche that facilitates the establishment of infection. However, by 3 weeks after infection, infected AMs can exhibit a pro-inflammatory response5. Recent single-cell RNA sequencing analyses revealed that there are multiple AM subpopulations after infection, some of which mount a pro-inflammatory response and impose stress on the bacilli3. In addition, another study implicated AMs as an M. tuberculosis-restrictive cell type following their migration out of the airways into granulomas25. Overall, the data support the idea that AMs exert little control over M. tuberculosis during the first 2 weeks of infection; however, some AM populations may be restrictive, particularly as the infection progresses and an adaptive immune response develops.
Over time, the bacilli diversify their niche by infecting polymorphonuclear neutrophils (PMNs), DCs and a variety of tissue-resident and recruited macrophage populations. Although PMNs eradicate a wide variety of microorganisms with reactive oxygen species (ROS) and neutrophil extracellular traps (NETs), M. tuberculosis resists ROS-mediated and NET-mediated killing26,27, and PMNs create a permissive niche for M. tuberculosis replication28,29,30. M. tuberculosis induces necrosis of infected PMNs, which promotes further M. tuberculosis growth following their phagocytosis by macrophages, which in turn recruits even more PMNs31,32. In humans, PMNs are implicated in lung immunopathology and the failed immune response that is characteristic of active TB33. On the other hand, during initial infection, PMNs promote CD4+ T cell priming in mice34. Thus, PMNs may mediate an indirect role in protection early in M. tuberculosis infection but overall appear to exert little direct antimycobacterial activity and are strongly associated with disease progression. Monocyte-derived DCs have been found to play a key role in transporting M. tuberculosis antigens from the lung to the draining lymph node, where conventional DCs present antigens to naive T cells35. Conventional lung DCs can be separated into two major populations on the basis of the surface markers CD11b and CD103. Both DC populations become infected with M. tuberculosis and exhibit delayed migration to the draining lymph node, but they differ in their capacity to prime naive T cells36,37. More recently, eosinophils have been shown to be recruited to the M. tuberculosis granuloma and were found to be protective against M. tuberculosis infection in mice38.
After infected AMs migrate into the lung interstitium, the bacilli infect additional populations of macrophages. The myeloid cells that become infected have been difficult to classify as they are recruited, proliferate, respond to stimuli and differentiate into macrophages and DCs in the inflammatory environment of the M. tuberculosis-infected lung. Aside from AMs, there are additional CD11c+ populations (which have been classified as either DCs or macrophages), as well as CD11clow/int populations (called 'interstitial macrophages' or 'recruited macrophages'2,5,6,7,36) that become infected. In mice, selective depletion of circulating monocytes using intravenous clodronate increases lung M. tuberculosis burden, suggesting the importance of monocyte-derived macrophages in M. tuberculosis control2. In addition, transcriptomic analysis of human monocyte-derived macrophages revealed that they generate a more robust inflammatory response to M. tuberculosis than AMs39. Thus, a prevailing idea is that monocyte-derived, recruited macrophages are more restrictive than AMs. However, recent data suggest a more complex picture. Single-cell RNA sequencing suggests there are at least four distinct M. tuberculosis-infected interstitial macrophage populations3. By 6 weeks after infection, a subset of CD11c+ monocyte-derived macrophage-like cells becomes the predominantly infected cell type, with up to 30% of this population in the lung infected5. The bacilli within this cell population also appear to experience less stress, suggesting that this CD11c+ macrophage population is more permissive than other monocyte-derived populations3. In a zebrafish larva model, Mycobacterium marinum selectively recruits permissive macrophages40. M. tuberculosis may also promote the recruitment of permissive macrophages as a consequence of manipulation of myelopoiesis and epigenetic reprogramming41 (Box 1). Thus, as suggested above for AMs, different interstitial and recruited macrophages may differ in the degree to which they are able to control M. tuberculosis replication. Interestingly, differences in the antimicrobial capacity of distinct macrophage populations are associated with different metabolic phenotypes (as discussed in more detail later), with AMs broadly appearing more committed to oxidative phosphorylation and interstitial macrophages broadly appearing more committed to glycolysis2. As macrophage control of M. tuberculosis appears to depend on direct interaction with CD4+ T cells42, some of the more restrictive macrophages may be those that have productively engaged with T cells. The extent to which the distinct myeloid populations are epigenetically preprogrammed is an area of investigation3. Whether some individuals are able to sterilize the infection during the innate immune phase is unknown. Interestingly, there is evidence that monocytes from some individuals who appear resistant to M. tuberculosis infection have altered immunometabolic responses43.
To conclude, M. tuberculosis is remarkably effective at disarming the innate immune response. It appears to grow readily in AMs, which deliver the bacilli to the lung interstitium, where additional phagocytes support expansion of the bacterial niche and dissemination from the site of infection. With the onset of adaptive immunity, some myeloid cell subsets appear to restrict bacterial growth, whereas others continue to provide a permissive niche. Interestingly, in mice that have been vaccinated with the attenuated vaccine (Mycobacterium bovis bacillus Calmette–Guérin (BCG)) before M. tuberculosis infection compared with unvaccinated mice, there is earlier transfer of bacilli from AMs into other macrophage populations and PMNs44. Strategies to shift the balance of infection towards restrictive macrophages or to enhance the antimycobacterial capacity of the permissive subsets might enable novel host-directed therapies (HDTs) to promote bacterial clearance. In addition, there is intense interest in understanding how these innate responses could be 'trained' to generate enhanced protection as part of vaccine strategies45 (Box 1).
Mechanisms of macrophage control
Macrophages detect a variety of M. tuberculosis pathogen-associated molecular patterns and respond by activating antimicrobial pathways. Cell surface and intracellular pattern-recognition receptors that respond to M. tuberculosis infection include Toll-like receptors (TLRs), C-type lectin receptors, NOD-like receptors and cyclic GMP–AMP synthase (cGAS)–STING, which drive macrophage transcriptional responses and modulate intracellular trafficking. Additionally, phagocytic receptors such as Fcγ receptors, complement receptors and scavenger receptors promote mycobacterial uptake (reviewed in ref.46). Detection of M. tuberculosis pathogen-associated molecular patterns by TLR2 and C-type lectin receptors activates NF-κB signalling, resulting in the production of pro-inflammatory cytokines, such as TNF and IL-6, as well as IL-1β and IL-18, which are processed to mature proteins by the NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome. Some host pattern-recognition receptors actually restrain pro-inflammatory responses, such as DC-SIGN, a major M. tuberculosis-binding C-type lectin receptor, which favours M. tuberculosis replication in macrophages47. These early host–pathogen interactions also influence downstream intracellular trafficking pathways. For instance, Fcγ receptor-mediated phagocytosis directs the bacteria to lysosomes, whereas mannose-receptor engagement inhibits this process48. In addition, in humans, M. tuberculosis-specific antibodies have been found that differ in their functional profiles and glycosylation patterns, and these antibodies impact lysosomal maturation and inflammasome activity49. How signals from these different pathways are integrated when individual bacilli engage multiple macrophage receptors, the degree to which different receptors operate in distinct myeloid cells and whether M. tuberculosis differentially regulates engagement of pattern-recognition receptors at different times of its life cycle are all open questions.
Once bacilli are internalized, macrophages have many ways to eliminate bacteria: restricting essential nutrients, such as iron; intoxication with heavy metals; antimicrobial peptide production; generation of reactive oxygen and nitrogen intermediates; and progressive phagosome acidification and lysosomal fusion50. Fighting off infection also induces a major overhaul of host metabolism to provide ATP and NADPH to fuel antimicrobial pathways. Macrophages switch to an inflammatory phenotype characterized by a Warburg-like shift to glycolysis, which contributes to M. tuberculosis growth restriction as shown in bone marrow-derived macrophages, mice and non-human primates (NHPs)2,51,52. The inflammatory metabolic phenotype is associated with remodelling of the tricarboxylic acid cycle, which enhances IL-1β production53,54, and supports production of itaconate55, which limits inflammatory responses and can inhibit enzymes of M. tuberculosis central carbon metabolism56,57. Although IL-1β is host-protective during early stages of TB, as the disease progresses, it can enhance pro-inflammatory eicosanoid production, resulting in an influx of neutrophils and exacerbating tissue pathology58,59. In the case of human pulmonary TB, a recent study of patients with untreated multidrug-resistant M. tuberculosis found that IL-1β and pro-inflammatory eicosanoids correlated with tricarboxylic acid cycle remodelling60. Mitochondria are central to host immunometabolism as they house multiple metabolic pathways, impact cell death and inflammasome activation, and produce ROS61. M. tuberculosis also induces the accumulation of fatty acids and cholesterol in lipid droplets by modulating the expression of host factors such as miR-33 and PPARα62,63,64. Host lipid droplets play a role in immune defence, and they are also metabolized by M. tuberculosis as a carbon source and used to build virulence lipids necessary for pathogenesis62,65,66. Thus, macrophages respond to infection by profoundly changing their metabolism, and M. tuberculosis appears to take advantage of the changes to its benefit.
Mycobacterial immune evasion strategies
Decades of work has been directed at defining how M. tuberculosis resists and impairs the myriad of macrophage defences. Its ability to impair phagolysosomal fusion was documented as early as 1971 (ref.67). We now appreciate that M. tuberculosis survives within diverse intracellular environments (Fig. 3). We also have an increasing understanding of the protein and lipid effectors that M. tuberculosis uses to undermine distinct host lysosomal trafficking pathways, as briefly discussed here and reviewed in detail recently68. NdkA prevents recruitment of endosomal markers such as RAB5 and RAB7, and SapM dephosphorylates phosphatidylinositol 3-phosphate69. PknG, a serine/threonine protein kinase, targets the RAB GTPase RAB7L1 to inhibit phagosome maturation, and more recently has been described to function as an unusual ubiquitin ligase that interferes with NF-κB signalling70,71. A variety of M. tuberculosis lipids, including phosphatidylinositol mannosides, lipoarabinomannan, diacyltrehaloses, polyacyltrehaloses, trehalose dimycolates and sulfoglycolipid 1, modulate inflammatory signalling and have also been implicated in arresting phagosome maturation, as recently reviewed68. The M. tuberculosis proteins NdkA, CpsA and PPE2 impair acquisition of NADPH oxidase on mycobacterial phagosomes, whereas M. tuberculosis catalase (KatG) detoxifies ROS72,73,74,75. By blocking NADPH oxidase, M. tuberculosis impairs an autophagy-related pathway called 'LC3-associated phagocytosis' which normally traffics microorganisms to the lysosome. M. tuberculosis NuoG can also detoxify phagosomal ROS and thereby limit subsequent TNF-mediated apoptosis76. M. tuberculosis encodes five type VII secretion systems (ESX-1–ESX-5) that export substrates out of the cell. ESX-1 and ESX-3 effectors play prominent roles in macrophage interactions and are required for virulence77. EsxA (exported by ESX-1) and PDIM (a cell envelope lipid) promote damage to the phagosomal membrane78,79,80,81,82, although exactly how remains unclear. Permeabilizing the phagosome enables M. tuberculosis to access nutrients and deliver effectors to the cytosol. In addition to modulating lysosomal trafficking, M. tuberculosis effectors inhibit the AIM2 and NLRP3 inflammasome and promote host cell necrosis83,84,85 (reviewed in ref.68). Phagosomal damage sets off a complex series of host–pathogen attacks and counterattacks. Macrophages can repair damage to the endolysosomal system, but the M. tuberculosis ESX-3 effector EsxH interferes with repair86. Macrophages recognize damaged phagosomes and attempt to route bacteria to the lysosome through selective autophagy. Damaged phagosomes expose glycan residues on intraluminal proteins, which are then bound by galectins, whereas ubiquilin 1 and the ubiquitin ligases parkin and SMURF1 promote ubiquitin deposition around the bacilli69,87,88. Galectin-tagged and ubiquitin-tagged mycobacteria are recognized by adaptor proteins such as p62, NDP52 and TAX1BP1 and are targeted for autolysosomal degradation. Bacterial and mitochondrial DNA that enter the cytoplasm activate the cGAS–STING pathway to promote production of type I interferons and activate autophagy89,90. Members of the M. tuberculosis PE-PGRS protein family block autophagy91,92, and, at least in human lymphatic endothelial cells, cytosolic mycobacteria aggregate to form cords, which are resistant to selective autophagy93. In addition, unlike infection with other bacilli, M. tuberculosis infection does not generate substantial mitochondrial ROS, which also appears to contribute to impaired NADPH oxidase activity and autophagy94. The ability of M. tuberculosis to block lysosomal trafficking pathways is overcome in part if macrophages are stimulated with interferon-γ before infection. Interferon-γ has a major impact on macrophage physiology and phagosome biology, as discussed further later. Finally, M. tuberculosis neutralizes the pH of the phagosome by producing an unusual terpene nucleoside (1-tuberculosinyladenosine) and also has mechanisms to resist killing by acidification95,96. As a final resort to eliminate intracellular infection, the host cell initiates apoptosis, a programmed cell death pathway, in which degraded cellular contents are retained inside membrane blebs (reviewed in ref.97). An apoptotic mode of cell death, combined with subsequent efferocytosis, is host protective98; however, M. tuberculosis induces necrosis using factors such as CpnT, PDIM and iron overload to favour bacterial dissemination68,81,83,99,100,101,102. Excessive tissue inflammation by type I interferons and TNF also contributes to M. tuberculosis-induced macrophage death61,103. Overall, M. tuberculosis takes a multifaceted approach to undermine macrophage antimicrobial responses and survive in diverse intracellular environments.
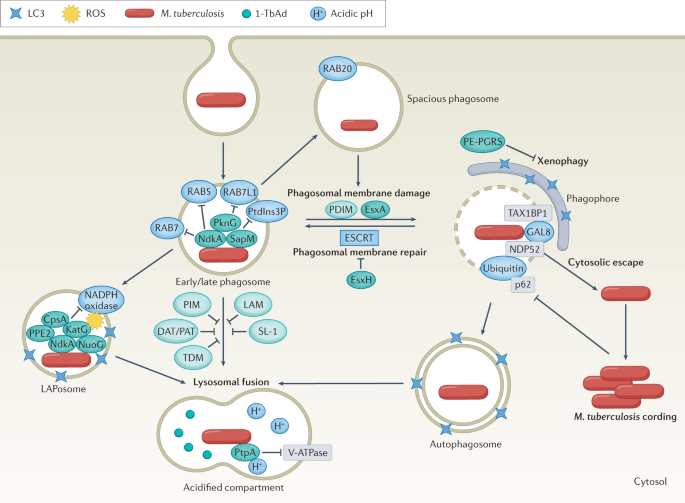
Mycobacterium tuberculosis is taken up in a single membrane-bound phagosome, which is targeted by the host to promote bacterial clearance. The interactions between host proteins (blue) and M. tuberculosis virulence factors (proteins in dark green; lipids in light green) shape infection outcomes. Sequential recruitment of molecular markers such as RAB5, RAB7 and phosphatidylinositol 3-phosphate (PtdIns3P) normally promote maturation of early to late phagosomes, but M. tuberculosis actively evades phagosomal maturation. In a process called 'LC3-associated phagocytosis' (LAP), NADPH oxidase assembles on the M. tuberculosis phagosome (LAPosome) and induces oxidative stress, but M. tuberculosis effectors impair the recruitment of the NADPH oxidase. M. tuberculosis can also be targeted to spacious phagosomes in a RAB20-dependent manner. In addition, the M. tuberculosis ESX-1 substrate EsxA and phthiocerol dimycoserosate (PDIM) promote phagosomal damage. M. tuberculosis EsxH inhibits the host endosomal sorting complex required for transport (ESCRT) machinery to prevent membrane repair. M. tuberculosis escapes into the cytosol and can replicate to form cords. The host attempts to recapture cytosol-exposed M. tuberculosis in double-membrane autophagosomes, which is inhibited by effectors, including PE-PGRS family members. M. tuberculosis contained within single-membrane or double-membrane compartments is targeted for lysosomal killing. M. tuberculosis has mechanisms to resist acidification, such as blocking vacuolar-type ATPase (V-ATPase) and producing the antacid 1-tuberculosinyladenosine (1-TbAd) to neutralize the pH. DAT, diacyltrehalose; LAM, lipoarabinomannan; PAT, polyacyltrehalose; PIM, phosphatidylinositol mannoside; ROS, reactive oxygen species; SL-1, sulfoglycolipid 1; TDM, trehalose dimycolate.
Our molecular understanding of how M. tuberculosis undermines macrophage functions comes largely from ex vivo studies, although there are efforts to develop more physiologically relevant systems12,104. As discussed earlier herein, in vivo macrophages differ on the basis of ontogeny, tissue residence and inflammatory environment. They are exposed to the alveolar lining fluid and undergo epithelioid transformations, form multinucleated giant cells and become lipid-laden and 'foamy'. How ex vivo macrophages (most often bone marrow-derived or monocyte-derived macrophages) recapitulate the diverse macrophage subsets in vivo is poorly defined. Whether M. tuberculosis preferentially resides in particular intracellular compartments in particular macrophage populations is not known. M. tuberculosis could also selectively deploy effectors to differentially manipulate macrophages on the basis of the macrophage state. There are several notable examples in which the findings of the ex vivo studies and in vivo data appear discordant. For example, numerous autophagy proteins contribute to macrophage control of M. tuberculosis ex vivo, but they are dispensable in vivo28, and NLRP3 is essential for M. tuberculosis-induced inflammasome activity in macrophages ex vivo but is expendable for IL-1β production in vivo105. In addition to differences between macrophages, these discrepancies could reflect compensatory host pathways in vivo or differences in the physiology of bacilli grown in vivo compared with liquid culture. For example, in vivo M. tuberculosis has access to host cholesterol and fatty acids, which impact virulence lipids of M. tuberculosis, such as PDIM66. Moreover, in ex vivo studies, investigators routinely generate single-cell suspensions of M. tuberculosis before macrophage infections, which could impact macrophage interactions. Although future work will have to assess the relevance of individual effectors in distinct cell types and during different stages of the life cycle of M. tuberculosis, overall, these studies have yielded substantial insight into molecular mechanisms that enable M. tuberculosis to disrupt macrophage functions. Understanding these immune evasion strategies of M. tuberculosis lays the groundwork for HDTs aimed at enhancing mycobacterial clearance, as discussed in more detail later.
Withstanding adaptive immunity
Mechanisms of host control
An effective adaptive immune response is required to prevent progressive, disseminated TB. Granulomas are the histopathological hallmark of M. tuberculosis infection. Mature granulomas are an organized collection of macrophages, neutrophils and lymphocytes. When there is an effective adaptive immune response, granulomas control and even sterilize the infection, becoming sclerotic and calcified, whereas active TB granulomas are necrotic and have a caseating appearance (like cheese). CD4+ T cells and TNF are crucial for well-organized granuloma formation and host protection106,107. Individuals with CD4+ T cell dysfunction from HIV infection or those treated with TNF inhibitors fail to form well-organized granulomas, and they are at high risk of developing active TB and disseminated infection8,46. CD8+ T cells are also activated during M. tuberculosis infection, and are detectable in the blood of human patients with TB108. Recent data support the idea that CD8+ T cells and CD4+ T cells synergize to control M. tuberculosis infection109. The role of B cells and humoral immunity in TB is less well established than that of T cells. Historically, B cell depletion studies failed to definitively establish a role for B cells or antibodies in M. tuberculosis infection control, although recent studies have uncovered potentially protective antibodies in NHPs and humans after intravenous BCG vaccination15,110. Inducible bronchus-associated lymphoid tissue is ectopic lymphoid tissue that contains B cell follicles and is found in a variety of inflammatory lung diseases. The abundance of inducible bronchus-associated lymphoid tissue and its proximity to M. tuberculosis granulomas correlates with protection against TB111.
Interferon-γ has long been implicated as a key factor through which CD4+ T helper 1 (TH1) cells mediate protection112. Interferon-γ has a major impact on macrophage physiology and phagosome biology, including enhancing autophagy and promoting RAB20-dependent targeting to spacious phagosomes113,114. There are a number of differences in how interferon-γ enhances the antimicrobial activity of human macrophages as compared with murine macrophages. Interferon-γ induces the expression of immunity-related GTPases, which target intracellular pathogens for destruction115. In mice, this is a large family, whereas humans have only two immunity-related GTPases, and their expression is not interferon-γ dependent115. In mouse macrophages, but not human macrophages, interferon-γ increases nitric oxide production by enhancing expression of inducible nitric oxide synthase114. Nitric oxide has direct toxic effects on M. tuberculosis and reduces immunopathology in vivo30,58. In human cells, interferon-γ induces the expression of the antimicrobial peptide cathelicidin, which depends on vitamin D116. Despite these apparent species-specific differences in macrophage responses to interferon-γ, a deficient type II interferon response is associated with TB susceptibility in both mice and humans. In addition to potentiating the antimicrobial activity of macrophages, the susceptibility of interferon-γ-deficient hosts may also be explained by impaired myelopoiesis and a lack of trained, protective monocytes and macrophages117 (Box 1). Interferon-γ also represses the recruitment of M. tuberculosis-permissive PMNs by inhibiting IL-1 and 12/15-lipoxygenase30, and it limits the accumulation of terminally differentiated, non-protective CD4+ T cells in the lung vasculature118. Interferon-γ is particularly important to limit disseminated infection in the spleen, whereas excessive interferon-γ in the lung can be detrimental if it is not repressed by PD1119. Similarly, infection of mice lacking cyclophilin D, a mitochondrial matrix protein, led to increased T cell proliferation, with higher production of TNF and interferon-γ, with associated tissue damage and reduced survival120.
Interferon-γ-independent mechanisms also contribute to CD4+ T cell-mediated control in the lungs in ways that are not well understood121. Studies to define interferon-γ-independent mechanisms identified the TNF family member CD153, whose expression on M. tuberculosis-specific TH1 cells inversely correlates with bacterial burden within granulomas in mice and NHPs, and is required for the protective capacity of CD4+ T cells122. In humans, CD153 expression is higher in patients with latent TB than in patients with active TB123. Further defining protective T cell subsets on the basis of their effector capabilities, memory and activation status, and migratory potential may reveal T cell signatures that correlate with protection and can guide vaccine strategies124. In conclusion, CD4+ TH1 cells are crucial for the control of M. tuberculosis infection and to limit dissemination. Interferon-γ plays a key role in their function, but interferon-γ-independent mechanisms also contribute to M. tuberculosis infection control in ways that are not well defined.
How M. tuberculosis undermines adaptive immunity
Although a robust adaptive immune response to M. tuberculosis develops, it is delayed and fails to sterilize the infection. In animal models, it takes several weeks before antigen-specific T cells are detected in the lungs. Similarly, in humans, it takes 2–8 weeks before TB-specific T cell responses are detectable. M. tuberculosis delays T cell priming by impairing DC maturation and interfering with efficient antigen presentation through a variety of mechanisms, as recently reviewed125 (Fig. 4). For example, infected DCs export M. tuberculosis antigens through kinesin 2-dependent vesicular transport to divert them from major histocompatibility complex class II presentation, making them less effective at activating T cells than uninfected DCs that take up M. tuberculosis antigens126. The cell envelope-associated serine protease Hip1 cleaves the chaperone GroEL2, which is strongly immunogenic in its full-length form, to prevent its presentation by DCs127. Several effectors that undermine phagosome integrity and phagosome maturation have also been shown to impair T cell priming. For example, EsxH impairs antigen processing by inhibiting the endosomal sorting complex required for transport (ESCRT) machinery128, PE_PGRS47 impairs antigen presentation by inhibiting autophagy92, and PDIM inhibits CD86 and IL-12p40 expression in infected DCs129. In addition, as mentioned earlier, NuoG, which inhibits apoptosis of infected neutrophils, delays antigen acquisition by DCs and trafficking to the lymph nodes130. It is possible to experimentally overcome the delay in T cell priming by direct pulmonary installation of M. tuberculosis antigen-treated DCs at the time of infection131. This leads to earlier recruitment of antigen-specific T cells to the lungs and more immediate control of M. tuberculosis replication. Overall, the delay in the adaptive immune response allows M. tuberculosis to establish infection, spread from the initial site of infection and grow relatively unhindered for weeks.
